The optical system of a flow cytometer consists of an elegant coordination of many components that function concordantly and synchronously to generate the signals that we need to measure in order to shed light on the biology at hand.
Understanding the optical system of a flow cytometer may seem unnecessary for performing a typical experiment, but the more you know about your instrument, the better you will be at understanding nuanced aspects of your data, as well as troubleshooting any potential issues that may arise during an experiment.
Commonly used component for flow cytometers:
- Lasers illuminate the stream with coherent, focused light of specific wavelength (energy) and power. This illumination facilitates the generation of fluorescence signals from cells labeled with fluorophores and light scatter signals from redirected laser light.
- Lenses focus laser light and collect light scatter and fluorescence optical signal, and direct this signal to the optical detection path.
- Mirrors are responsible for directing light through the detection path and partitioning it so that fluorescence and scattered light are directed to the appropriate detectors.
- Filters, placed in front of detectors, function to restrict the light that is introduced to the PMT detectors so that each detector can be dedicated to measuring fluorescence from a specific set of fluorophores.
The specificity of detection in Flow cytometers is controlled by optical filters, which block certain wavelengths whilst transmitting (passing) others.
The most used filters are fluorescence emission filters and dichroic filters which are specifically designed to isolate the relatively weak emission signals of the target fluorophore for each channel while blocking out the signals of neighboring fluorophores.
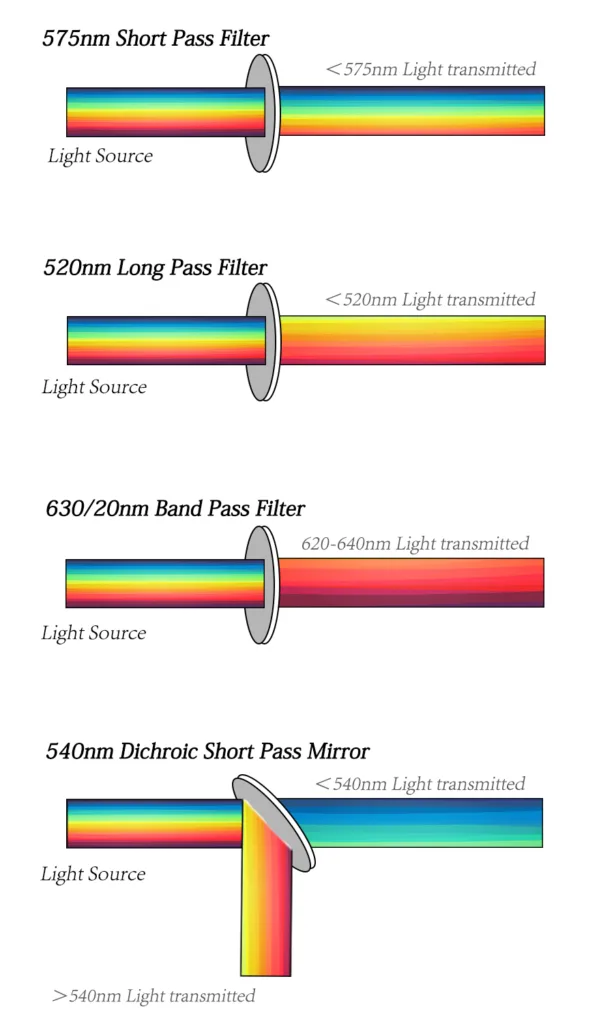
There are two major filter types. The dichroic filters can block light by phased reflection allowing certain light to pass through and interfering with other wavelengths. Placed at 45° relative to the direction of incident or oncoming light, dichroic mirrors come in a long pass and short pass flavors. A 600 LP (long pass) mirror, for example, reflects light shorter than 600 nm while allowing light longer than 600 nm to pass through. A 600 SP (short pass) would do the opposite. Long pass filters allow light through above a cutoff wavelength; short pass filters permit light below a certain wavelength. Bandpass filters transmit light within a specified narrow range of wavelengths (termed a bandwidth), including narrow bands within a 30nm bandwidth called narrow bandpass filters and board bands over 30nm filters called board bandpass filters.
Lasers are the most commonly used light sources in flow cytometry. Lasers produce a single wavelength of light (a laser line) at a specific frequency. They are available at different wavelengths ranging from ultraviolet to far red and have a variable range of power levels (photon output/time typically specified in mW).Common fluorescent groups of lasers:
| Laser | wavelengths | Common fluorescent groups of lasers |
| UV | 355 nm | DAPI, Hoechst, LIVE/DEAD Blue, Brilliant Ultraviolet |
| Violet | 405–407 nm | Pacific Blue, eFluor 450, Pacific Orange, eFluor 506, Super Bright 436, Super Bright 600, Brilliant Violet, LIVE/DEAD Yellow, LIVE/DEAD Aqua, LIVE/DEAD Violet, CFP |
| Blue | 488 nm | FITC, Alexa Fluor 488, Dylight 488, PE, PE tandems, PerCP, PerCP tandems, PI, 7AAD, eGFP, YFP |
| Green | 532 nm | PE, PE tandems, Alexa Fluor 532, PI, mCherry, dTomato, RFP |
| Yellow | 561-568 nm | PE, PE tandems, PI, mCherry, dTomato, RFP |
| Red | 633-647 nm | APC, Alexa Fluor 647, Alexa Fluor 700, APC tandems |
